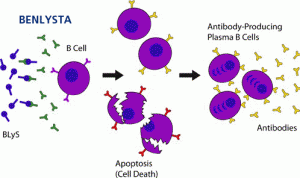
Historic and Hopeful Day for Patients living with Lupus – FDA Advisory Committee’s Recommendation to Approve Benlysta for Lupus
The U.S. FDA Arthritis Advisory Committee voted 13 to 2 to recommend approvalof the treatment developed by Human Genome Sciences (HGS) and GlaxoSmithKline
Tuesday, November 16, 2010
The Lupus Research Institute (LRI) and its National Coalition of state and local lupus organizations are pleased with the U.S. Food and Drug Administration (FDA) Advisory Committee’s vote to recommend approval of Benlysta® for active systemic lupus erythematosus (SLE), offering patients new found hope for the first approved treatment option in more than 50 years.
The U.S. FDA Arthritis Advisory Committee voted 13 to 2 to recommend approval of the treatment developed by Human Genome Sciences (HGS) and GlaxoSmithKline. The FDA will take the Advisory Committee’s vote into consideration as it reviews Benlysta for approval. A decision by the FDA is expected to be announced by December 9, 2010.
“The panel’s recommendation may signal the historic breakthrough that lupus patients have long awaited,” said Margaret G. Dowd, president of the LRI, an organization uniquely dedicated to funding innovative approaches to lupus research. “This positive vote reinforces our community’s resolve to increase the pace of scientific discovery and clinical development in lupus, made possible by the active participation of lupus patients in clinical trials.”
“The results of both of Benlysta’s phase III trials, BLISS-52 and BLISS-76, show that it reduces SLE disease activity, has a favorable safety profile and allows lupus patients to reduce their use of current treatment options that can cause serious adverse effects,” said Benjamin Schwartz, M.D., Ph.D., a professor of clinical medicine at Washington University School of Medicine in St. Louis and a member of the LRI Scientific Advisory Board.“Benlysta would be a welcome addition to the limited number of treatments available for people with lupus.”
“It’s exhausting to be on such a tedious schedule of medicines which cause so many side effects,” said lupus patient, Sabrina Nixon, “If approved, Benlysta will hopefully decrease the number of medications patients like me will need to stay alive.”
Lupus is a perilous and chronic autoimmune disease that impacts an estimated 1.5 million Americans, primarily young women in their childbearing years. In lupus, the immune system attacks the body’s own tissues and vital organs, making the illness a leading cause of premature cardiovascular disease, heart attack, stroke, and kidney disease among young women. Finding the cause, the cure and new treatments for lupus is a complex and challenging process.
source: http://lupusresearchinstitute.org Founded in 2000, the Lupus Research Institute is the nation’s only nonprofit organization solely dedicated to driving novel research in lupus. The LRI funds innovative and creative research to discover new scientific solutions for the disease.
Some more information on the topic:
- For more information about the FDA committee’s meeting today:
- on FDA.gov please click here. (please note that this is a large pdf file)
- Lupus Alliance of America finds some of the side effects of Benlysta worth noting.
- LFA’s reaction to todays panel.
- The New York Times weighs in on the FDA panel’s support of Benlysta
- Here is a direct link to Humane Genome Sciences web page about Belysta.
- This is a link to how BenLysta works
___________________________________________________________
From Christine:
How do I feel about the news about BenLysta? I feel hope for the first time in my life with lupus. I am aware that this is nota cure, but a new treatment. But the fact that it is a new treatment for the first time in over 50 years excites me. The thought that it can change my quality of life, change how I can care for my family, and care for myself… is almost too much to imagine. I need to take pills 4-5 times a day. Over the course of the day that equals 18 pills. I don’t even want to do the math of what that is over a lifetime! If Benlysta can help lower or take away any of these toxic medications, I will be happy. If it doesn’t work for me, but does for others in the Lupus community, I am happy. I see this as the first HUGE step in scientists, government, pharmaceutical companies and doctors taking Lupus seriously and starting to focus their time and energy not just on Lupus but on all autoimmune diseases. This could be the start of something great. Hey, Lupus is in the news.. that’s always a plus. Whenever a product comes out, you know that down the road their will be “competitive” companies trying to do better, or cheaper versions of this drug, or new drugs for lupus. Today’s hearing broke down years of the same old treatments, with the same nasty side effects. I hope my daughter knows a totally different life with lupus then I know now. I hope she gets to know her mother even better, since maybe I will feel even better. Maybe just maybe, this drug will be approved, the people living with lupus can receive it, and lives can start changing. A girl can dream can’t she?
Over all I am happy, I am hopeful, and I am excited for what the future holds and I thank the researchers and scientists behind Benlysta for making this drug a priority.
-
Teri Jackson
-
Steph
-
westomoon
-
Kristy Howard
-
Shannon – Fibro Warrior
-
Jen Martin
-
clara elliott